Iredia D. Iyamu

- Assistant Professor
- Department of Medicinal Chemistry
Contact Info
Lawrence
1567 Irving Hill Rd
Lawrence, KS 66045
Education —
Research —
Chemical probes are essential tools in elucidating complex cellular processes that drive cancer and other diseases. Additionally, a probe that displays a promising effect can serve as a beginning chemical compound for drug discovery.
The Iyamu laboratory is focused on:
- Developing chemical probes for potential disease targets for utility in target validation and drug discovery with a focus on epigenetics
- Utilizing high-throughput and fragment-based lead discovery screening to discover chemical leads.
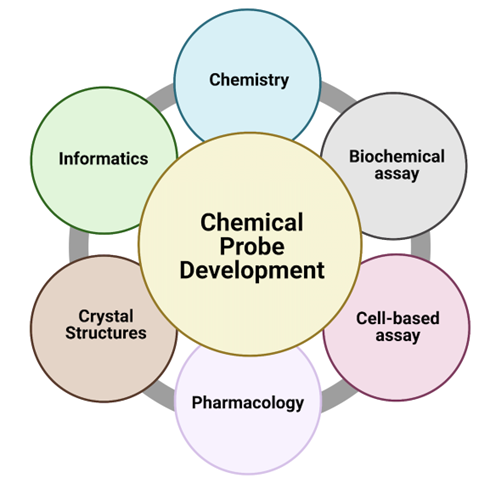
Research in the Iyamu group will develop chemical probes for novel biological targets through an integrative approach that includes the application of chemistry in conjunction with informatics (bio- and cheminformatics) and molecular interaction studies for probe identification and optimization. We will also use biochemical assays to investigate in vitro activity, cell-based assays to validate cellular target engagement, and pharmacology to explore the functions of the probe in developing new therapeutic strategies. We are working at the interface of chemistry and biology to design tools and inhibitors to study the function of human proteins in cells and diseases.
Research interests:
- Epigenetics
- Posttranslational modification
- Methyltransferase
- Acetyltransferase
- Medicinal chemistry
- Drug discovery
Selected Publications —
- Meng, Y.; Iyamu, I. D.; Ahmed, N. A.; Huang, R. Comparative Analysis of Two NNMT Bisubstrate Inhibitors through Chemoproteomic Studies: Uncovering the Role of Unconventional SAM Analogue Moiety for Improved Selectivity. ACS Chem Biol. 2024, 19 (1), 89-100.
- Deng, Y.; Song, X.; Iyamu, I. D.; Dong, A.; Min, J.; Huang, R. A unique binding pocket induced by a noncanonical SAH mimic to develop potent and selective PRMT inhibitors. Acta Pharm Sin B. 2023, 13 (12) 4893-4905.
- Iyamu, I. D.; Zhao, T.; Huang, R. Structure-Activity Relationship Studies on Cell-potent Nicotinamide N‐Methyltransferase Bisubstrate Inhibitors. Journal of Medicinal Chemistry 2023, 66 (15), 10510-10527.
- Song, Q.; Wang, J.; Griffiths, A.; Lee, S. M.; Iyamu, I. D.; Huang, R.; Cordoba-Chacon, J.; Song, Z. Nicotinamide N-methyltransferase upregulation contributes to palmitate elicited peroxisome proliferator-activated receptor transactivation in hepatocytes. American Journal of physiology. Cell Physiology, 2023, 325 (1) C29-C41.
- Nacheva, K.; Kulkarni, S.; Kassu, M.; Flanigan, D.; Monastyrskyi, A.; Iyamu, I. D.; Doi, K.; Barber, M.; Namelikonda, N.; Tipton, J.; Parvatkar, P.; Wang, H.; Manetsch, R. Going Beyond Binary: Rapid Identification of Protein–Protein Interaction Modulators Using a Multi-Fragment Kinetic Target-Guided Synthesis Approach. Journal of Medicinal Chemistry, 2023, 66 (7), 5196-5207.
- Deng, Y.; Song, X.; Iyamu, I. D.; Dong, A.; Min, J.; Huang, R. A unique binding pocket induced by a noncanonical SAH mimic to develop potent and selective PRMT inhibitors. Acta Pharmaceutica Sinica B, 2023.
- Zhou, J.; Deng, Y.; Iyamu, I. D.; Horton, J.; Yu, D.; Hajian, T.; Vedadi, M.; Rotili, D.; Mai, A.; Blumenthal, R.; Zhang, X.; Huang, R.; Cheng, X. Comparative study of adenosine analogs as inhibitors of protein arginine methyltransferases and a Clostridioides difficile-specific DNA adenine methyltransferase. ACS chemical biology, 2023, 18 (4), 734-745.
- Iyamu, I. D.; Vilseck, J. Z.; Yadav, R.; Noinaj, N.; Huang, R. Exploring Unconventional SAM Analogues To Build Cell-Potent Bisubstrate Inhibitors for Nicotinamide N‐Methyltransferase. Angew. Chem. Int. Ed. 2022, 61, e202114813 .
- Iyamu, I. D.; Zhao, Y.; Parvatkar, R.; Roberts, B. F.; Casandra, D. R.; Wojtas, L.; Kyle, D. E.; Chakrabarti, D.; Manetsch, R. Structure-activity and structure-property relationship studies of spirocyclic chromanes with antimalarial activity. Bioorganic and Medicinal Chemistry, 2022, 57, 116629.
- Han, Y.; Iyamu, I. D.; Clutter, M. R.; Mishra, R. K.; Lyman, K. A.; Zhou, C.; Michailidis, I.; Xia, M. Y.; Sharma, H.; Luan, C.; Schiltz, G. E.; Chetkovich, D. M. Discovery of a small-molecule inhibitor of the TRIP8b-HCN interaction with efficacy in neurons. J. Biol. Chem. 2022 May 24;102069.
- Dong, G.; Iyamu I. D.; Vilseck, J. Z.; Chen, D.; Huang, R. Improved Cell-Potent and Selective Peptidomimetic Inhibitors of Protein N-Terminal Methyltransferase 1. Molecules. 2022; 27(4):1381.
- Hopkins, B.; Mashuo, I.; Ren, D.; Iyamu, I. D.; Lv, W.; Malik, N.; Martemyanov, K.; Schiltz, G. E.; Miller, R. Effects of Small Molecule Ligands on ACKR3 Receptors. Molecular Pharmacology, 2022. 102 (3): 128-138.
- Iyamu, I. D.; Al-Hamashi, A. A.; Huang, R. A pan-inhibitor for protein arginine methyltransferase family enzymes Biomolecules 2021, 11, 854.
- Griffiths,A.; Wang, J.; Song, Q.; Iyamu, I. D.; Liu, L.; Park, J.; Jiang, Y.; Huang, R.; Song, Z. Nicotinamide N-methyltransferase upregulation via the mTORC1-ATF4 pathway activation contributes to palmitate-induced lipotoxicity in hepatocytes. Am. J. Physiol. Physiol. 2021, 321, C585–C595.
- Iyamu, I. D.; Huang, R. Mechanisms and inhibitors of nicotinamide N-methyltransferase. RSC Medicinal Chemistry, 2021, 12 (8), 1254-1261.
- Iyamu, I.D.; Huang, R. Development of fluorescence polarization-based competition assay for Nicotinamide N‐Methyltransferase. Analytical Biochemistry. 2020, 604, 113833.
- Chen, D.; Li, L.; Diaz, K.; Iyamu, I. D.; Yadav, R.; Nionaj, N.; Huang, R. Novel Propargyl-Linked Bisubstrate Analogues as Tight-Binding Inhibitors for Nicotinamide N‐Methyltransferase. Journal of Medicinal Chemistry. 2019, 62, 10783−10797.
- Iyamu, I. D.; Lv, W.; Malik, N.; Mishra, R. K.; Schiltz, G. E. Discovery of a novel class of potent and selective tetrahydroindazole-based sigma-1 receptor ligands. Bioorganic and Medicinal Chemistry, 2019 27(9), 1824-1835.
- Iyamu, I. D.; Lv, W.; Malik, N.; Mishra, R. K.; Schiltz, G. E. Development of Tetrahydroindazole-Based Potent and Selective Sigma-2 Receptor Ligands. ChemMedChem, 2019,14,1248 –1256.
- Neha Malik; N.; Iyamu, I. D.; Scheidt, K. A.; Schiltz, G. Synthesis of a novel fused pyrrolodiazepine-based library with anticancer activity. Tetrahedron Letters, 2018, 59, 1513-1516.
- Blake, L. D.; Johnson, M. E.; Siegel, S. V.; McQueen, A.; Iyamu, I. D.; Shaikh, A. K.; Shultis, M. W.; Manetsch, R.; Kyle; D. E. Menoctone resistance in malaria parasites is conferred by M133I mutations in cytochrome b that are transmissible through mosquitoes. Antimicrobial Agents and Chemotherapy, 2017, 61, 8.
Roberts, B. F.; Iyamu, I. D.; Lee, S.; Lee, E.; Ayong, L.; Kyle, D. E.; Yuan, Y.; Manetsch, R.; Chakrabarti, D. Spirocyclic Chromanes Exhibit Antiplasmodial Activities and Inhibit All Intraerythrocytic Life Cycle Stages. International Journal for Parasitology: Drugs and Drug Resistance, 2016, 6, 85-92.